|
|
 |
| |
 |
 Research Research |
 |
| |
|
|
| 1. Molecular Mechanism of Myelination and Remyelination
Myelin sheath in the peripheral nerve is essential for the rapid conduction of nerve impulse along the long distance of axon. The abnormal development of myelin sheath due to genetic causes, such as Charcot Marie Tooth diseases, or epigenetic mechanisms results in clinical problems in our sensory and motor function of peripheral nerves. Thus, the researches on the molecular mechanisms of myelination provide an essential insight into the development of therapeutic strategy for these myelin-related diseases. |
| |
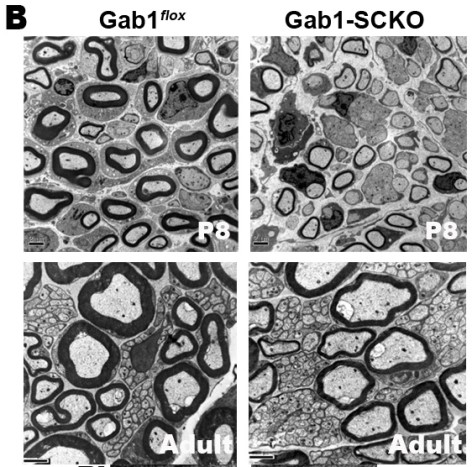 |
The myelination of peripheral nerves is achieved through an ensheathment process involving the extended membranes of Schwann cells after birth, and the thickness of the myelin sheath is proportional to the axonal diameter. To determine the ensheathment fates of axons and properly organize myelin sheath formation relative to axonal diameter, several molecular aspects of axon-Schwann cell interactions have been emphasized. Axonal signals, such as neuregulin-1 (NRG1)-type III, determine not only the ensheathment of large axons but also the extents of myelination. One of the our research goals is to understand the signaling mechanism of myelination by NRG1 and we recently reported that Grb2-associated binder 1 is an essential mediator for NRG1 signaling in peripheral nerve myelination and remyelination by using Schwann cell specific conditional knockout of Gab1 gene (Shin et al., 2014, J. Neurosci., Shin et al., Glia, 2017, Ghidinelli et al., Plos Biol., 2017). |
| We now are pursuing to find a novel Gab1-dependent genes and how Gab1 regulates the transcription factors responsible for myelin gene expression. In addition, the role of Gab1 and Gab2 in remyelination following nerve injury was under investigation. For this research, we employ tissue specific knockout technology, primary Schwann cell cultures, microarray, promoter assay and general Cell and Molecular biological techniques. |
| |
|
(Shin et al., 2014, J. Neurosci., Shin et al., Glia, 2017) |
| |
| |
| |
2. Molecular mechanism of Demyelination; The Concept of “Demyelinating Schwann cell” and serum biomarker development
The response of Schwann cells, a sole glia in peripheral nerve, to axonal injury is rapid and myelin degradation is initially performed by Schwann cells. We have revealed several important mechanisms of peripheral demyelination by Schwann cells. The actin polymerization by Schwann cells determine the stereotypic fragmentation of myelin sheath after nerve injury (Jung et al., 2011, J. Neurosci.). Soon, Schwann cells dissolve myelin sheath with help of lysosome and proteasome (Lee et al., 2009, Glia). We also have shown the role of Schwann cell autophagy in myelin clearance during Wallerian degeneration and inflammatory demyelinating neuropathies (Jang et al., Glia, 2016, 2017). By exploring the mechanism of demyelination by Schwann cells, we introduced novel concept of “Demyelinating Schwann cell” to emphasize the role of Schwann cells in the rapid clearance of the myelin sheath, which was made by themselves, during demyelination (Park et. al., Glia, 2018, CMLS, 2020). We are investigating how Schwann cell autophagy is regulated for demyelination and trying to develop novel tools to control demyelinating neuropathies. |
| |
|
| (Park et al., 2018, Glia) |
| |
In addition to this basic study, we are developing serum biomarkers reflecting the demyelinating Schwann cells in peripheral neuropathies. Schwann cells show very plastic phenotypic changes in several disease conditions, and by investigating the Schwann transcriptomes demonstrating disease-related changes, we reported several potential biomarkers of demyelinating neuropathies (Kim et al., Ann. Clin. & Trans. Neurol., Scientific reports, 2019). For these research, we utilize patient sera, sural nerve biopsies as well as several animal models of peripheral neuropathies such as inflammatory and hereditary neuropathies. We got an international patent on this biomarker technique and are trying to apply our potential biomarkers for clinical uses. |
| |
|
(Kim et al., Ann. Clin. & Trans. Neurol, 2019) |
| |
| |
| |
| |
| |
| |
|
| |
|
|
| |
|
|
|
Department of Molecular Neuroscience, Dong-A University: 32, Daesingongwon-ro, Seo-gu, Busan, Republicof Korea
Copy right ⓒ Hwan Tae Park all right reserved. Dong-A University.
|



