| 03 |
|
 |
 |
1. Ultraviolet Phtoelectron Spectroscopy (UPS)The UPS measures experimental molecular orbital energies for comparison with theoretical values from quantum chemistry, which was also extensively developed in the 1960s. The photoelectron spectrum of a molecule contains a series of peaks each corresponding to one occupied (valence-region) molecular orbital energy level. It is particularly sensitive to the surface region (to 10 nm depth), due to the short range of the emitted photoelectrons (compared to X-rays). It is therefore used to study adsorbed species and their binding to the surface, as well as their orientation on the surface. |
||
|
||
 |
||
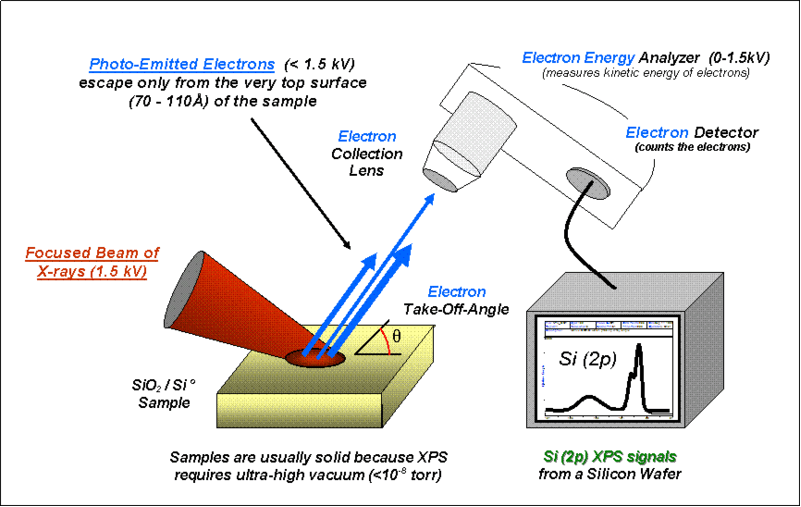
Basic components of a monochromatic XPS system. |
||
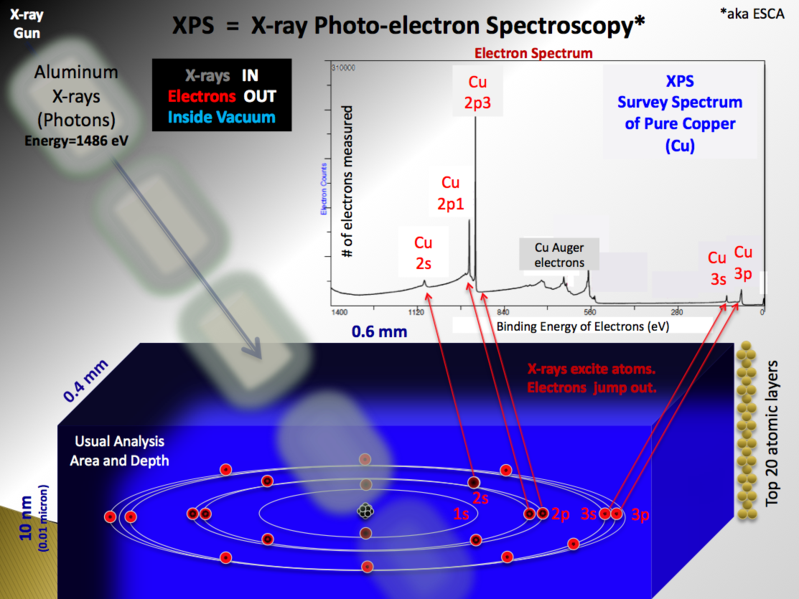
2. X-ray Photoemission Spectroscopy (XPS) XPS is a surface analysis technique and a quantitative spectroscopic technique that measures the elemental composition, empirical formula, chemical state and electronic state in materials that exist within a material. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 1 to 10 nm of the material being analyzed. XPS requires ultra high vacuum (UHV) conditions. |
||
 |
||
Rough schematic of XPS physics - "Photoelectric Effect. |
||
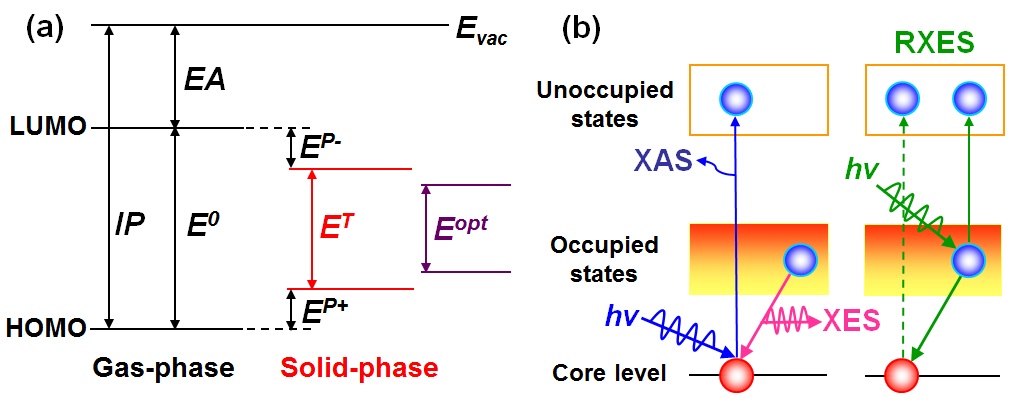
3. X-ray Absorption and Emission Spectroscopy (XAS and XES) Absorption of an X-ray photon promotes an electron from the core level to an unoccupied state, leaving behind a hole in the core level. XAS thus reveals information about LUMO levels. In XES, an emitted photon is detected when an electron in the occupied valence states refill a core hole, providing information on the HOMO levels. The use of resonant XES (RXES) allows also selecting specific atomic species in materials and probing local energy structure by tuning the incident photon energy to certain electronic transitions. |
||
 |
||
| a) Left: The ionization potential (IP) and electron affinity (EA) of a gas-phase molecule with an energy gap E0. Center: Relaxed polaron levels including the polarization energies EP+ for cations (“hole”) and EP for anions (“electron”) in a charged molecule. Right: the optical gap (Eopt) for the neutral excited molecule. | In XAS, a core electron is excited to the unoccupied states, when X-ray is incident. Here, the photons measured in XES are produced when electrons in the occupied states refill the core hole. The energy loss in RXES typically corresponds to the transitions in which electrons in the occupied states are scattered to the unoccupied states when excitation energy is tuned near or on-threshold. |
|
| Reference | ||
1. Seo, J. H.; Nguyen, T.-Q. J. Am. Chem. Soc. 2008, 130, 10042. 2. Seo, J. H.; Jin,Y.; Brzezinski, J. Z.; Walker, B.; Nguyen, T.-Q. ChemPhysChem 2009, 10, 1023. 3. Braun, S.; Salaneck, W. R.; Fahlman, M. Adv. Mater. 2009, 21, 1450. 4. Ishii, H.; Sugiyama, K.; Ito, E.; Seki, K. Adv. Mater. 1999, 11, 605. 5. J. Hwang, A. Wan, and A. Kahn, Mater. Sci. Eng. R. 2009, 64, 1. 6. http://en.wikipedia.org/wiki/X-ray_photoelectron_spectroscopy 7. http://en.wikipedia.org/wiki/Ultraviolet_photoelectron_spectroscopy |
||



